|
 |
|
Rummel's Current Assignments contains the dates and brief statements about the assignments.
Assignment details, handouts, and reference materials for each day are posted in the class website via secure log-in.
|
| |
|
|
|
| |
 |
|
History of
Chemistry Lecture
Outlines |
These web pages were generated using the free CMAP concept mapping software from the Institute for Human and Machine Cognition (IHMC) at the University of West Florida. The original cmaps are loaded on our class server as .zip files.
These mind maps help fill in the history of chemistry from the Copper Age till the time of Newton, where the "History of Chemistry" document takes over.
Names to know from the history of chemistry packet can be found here. |
As conceptual models progressed from Dalton's four postulates of 1803 to the quantum mechanical model of the 1920's, new concepts and vocabulary have been introduced in order to make sense of how these smallest units of elements behave.
|
|
| |
|
|
|
| |
 |
|
Chapter 14:
Intro to the Periodic Table |
|
- Chem4kids has basic review of atoms and elements.
- Webelements .com will give you all the info you need about any element on the Periodic Table.
|
|
| |
|
|
|
| |
 |
|
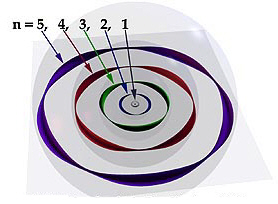
Chapter 15: The Quantum Atom |
|
- Make sure you understand why electrons only exist at whole number wave intervals called orbits around nucleus.
- Astronomers use Kirchoff's Spectral Analyzer every day. Sites that illustrate and discuss Emission Nebulae are:
|
|
|
|
|
|
| |
 |
|
Chapter 16: The Nucleus
|
- You should know these concepts:
- isotopes
- radioactive isotopes
- mass spectrometers
- decay products
- half-life
- fission vs. fusion
- E= mc2
|
|
|
|
|
| |
 |
|
Chapter 19: Bonding
 |
Types of solids
|
 |
Electronegativity
|
 |
Bond formation
|
|
PowerPoint Slide Presentation
- Outline notes may be printed out from the slide controls on the bottom of the frame
|
- Note: Requires high-speed internet connection for PowerPoint slide shows!
|
|
|
- A concept map (large one!) showing many of the general concepts covered.
|
|
| |
|
|
| |
 |
|
Chapter 20: Molecular Interactions
|
- Entire chapter covered except for 20.4
- "Lewis Models" allow you to predict the 3-D shapes of covalent molecules.
- The polarity of the molecule can then be determined by looking for unbalanced forces or net dipoles.
- The solubility, melting & boiling points can then be compared for various molecules or ionic crystals.
|
- Chapter 20 flash tutorials:
|
|
| |
|
|
|
|
| |
 |
|
Chapter 18: Mixtures, Separation |
- Will not cover 18.4-18.6
- Using the decision tree or flowchart for determining the type of matter (pure vs. type of mixture) in a sample.
|
|
|
| |
|
|
|
| |
|
|
Chapter 17 & 21:
Chemical Changes |
- Both entire chapters covered
- Distinguishing between physical and chemical properties
- Identifying chemical changes
- Identifying the types of chemical reactions and balancing the equations
|
|
|
|
|
|
|
| |
 |
|
Chapter 22:
Acids & Bases |
- Be able to define acids and bases as proton donors and acceptors
- Know the pH scale definition as the inverse exponent of the concentration of H+ ions in a solution (p. 542). Know that the pH is a log or 10x difference scale.
- Know the Universal Indicator colors that acids, bases, and neutral solutions formed in the lab.
- Know the general properties of acids and bases.
|
|
|
| |
|
|
|
|
| |
 |
|
Chapter 23: Redox Reactions |
- Distinguish oxidation and reduction processes
- Describe how batteries of all types utilize redox reactions
- Know the pros and cons of fuel cells
- Describe how our cell's mitochondria oxidize high energy carbon-hydrogen bonds in order to regenerate ATP
|
|
|
|
|
|
|
| |
 |
|
Chapter 24.1 & Biomolecules
|  Molecular models (for general viewing- you won't have to write any of these down!) are good for figuring out polarities. These molecules below use the free CHIME browser plug-in for Internet Explorer (requires registration) to display 3-D models: Molecular models (for general viewing- you won't have to write any of these down!) are good for figuring out polarities. These molecules below use the free CHIME browser plug-in for Internet Explorer (requires registration) to display 3-D models: |
- Sugars are polar, while lipids, being hydrocarbon rich, are nonpolar molecules.
- The amino acids in protein chains can be either polar or nonpolar based on their "R" side-group
- a -CH3 group would be nonpolar and a -COOH group would be polar.
- Polynucleotides are very polar due to their sugar-phosphate "backbones".
- Sugars and Lipids generally do not contain Nitrogen.
- Proteins (polypeptides) and polynucleotides both are built on nitrogen containing subunits.
- The basic unit of a protein is an amino acid while a nucleotide (nitrogen base + sugar + phosphate) is the basis for a polynucleotide.
| |
 Dr. Dave Woodcock's molecular models (choose a section) Dr. Dave Woodcock's molecular models (choose a section) |
 CMU small biomolecule models (a wide variety) CMU small biomolecule models (a wide variety) |
 Sugars Sugars
|
 Proteins or polypeptides Proteins or polypeptides
- amino acid: the basic monomer unit
- basic amino (-NH2) group
- acid (-COOH) group
- side group chain (-R) can be polar or nopolar
- amino acids arestrung together by peptide bonds to make primary structures
- secondary structures are created due to molecular attractions of the side groups to create:
- enzymes: teriary (globular) or quartenary (multiple globs) protein structures that help make or break bonds.
- proteins can also be
|
 Lipids or fats Lipids or fats
|
 Polynucleotides Polynucleotides
- DNA (double stranded helix)
- RNA (single stranded- can twist back on itself to form many different structures)
- ATP is a single nucleotide based molecule that is the "energy currency" of the cell.
|
| |
|
|
|
| |
 |
|
Online Practice Chemistry Exam |
- 70 questions covering the entire chemistry section
- Self-test with immediate feedback
In the book there is a final practice test on pg. 610. #1-9, 12-17, 18-34 are items we covered. |
- It is thoroughly recommended that these questions and their answers are used as part of your exam preparation.
|
|
| |
|
|
|
| |
 |
|
Chemistry Links Section |
Chemistry Web sites that you should visit to learn more about a topic, take online review/ practice over a unit, or find a different way to master a units topics.
|
|
|
| |
|
|
|
| |
|
|
|
|
|